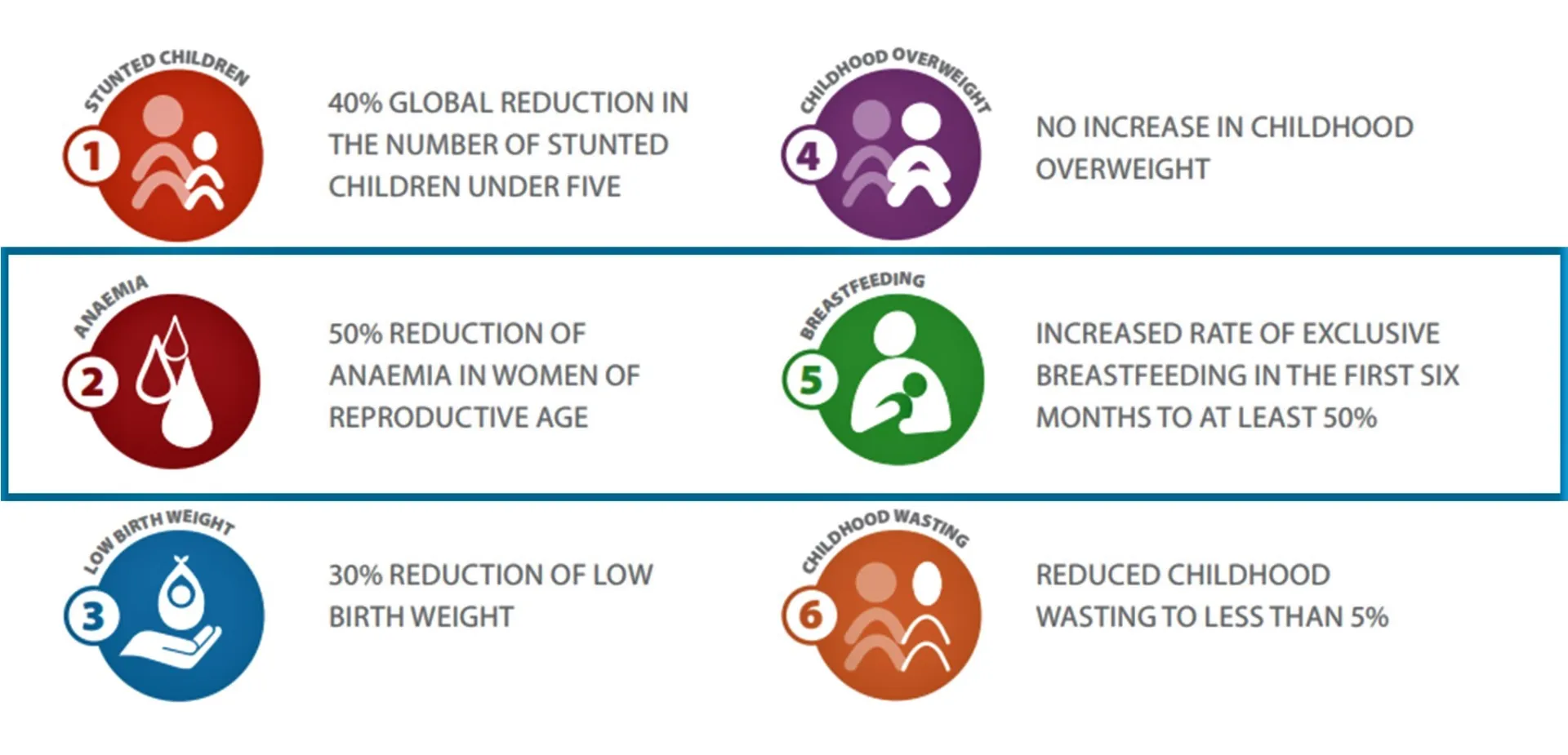
Last week the WHO hosted the 77th World Health Assembly (WHA). Back in 2012, the 65th WHA endorsed a set of six global maternal, infant and young child nutrition targets with an endline of 2025. In May 2024, a proposal was shared with the Member States about extending these nutrition targets to 2030 to match the Sustainable Development Goals (SDG).
WHO recently launched an online consultation to get feedback on their proposed targets for the six outcome indicators as well as supporting process indicators with operational targets. Comments on the discussion paper are being accepted from “Member States, civil society, academics, and other UN agencies” through 21 June 2024 here: https://bit.ly/3wWKmyS.
DataDENT’s expertise in coverage measurement is informing our feedback on the process indicators proposed by WHO for the anemia and exclusive breastfeeding (EBF) targets.
According to the WHO discussion paper, the proposed process indicators meet the following criteria:
- causally linked to the nutritional outcomes
- available data for at least 50 countries
- indicator can change relatively quickly in response to public health actions
- indicator is measurable on a continuum (i.e. not yes/no)
DataDENT recommends an additional criterion: indicator estimates should have acceptable levels of validity. Here we discuss evidence for validity of the proposed anemia and EBF process indicators.
Process indicators for the anemia target
The WHO proposed three process indicators for the anemia target: option 1 – percent of women consuming any iron-containing supplements during pregnancy, option 2 – percent of women consuming at least 90 iron-containing supplements during pregnancy, option 3 – percent of wheat or maize flour that is fortified with iron. The recommended source for options 1 and 2 are the Demographic and Health Surveys (DHS) and for option 3 the Global Fortification Data Exchange (GFDx).
DataDENT was a research partner for a 2022 validation study in Nepal which found that women responding to DHS-style questions could accurately report whether they received any iron folic acid (IFA) during their most recent pregnancy but could not accurately report how much they received. Three out of four women in the study (72.6%) overreported the number of IFA tablets they received, by an average of 70 tablets. A smaller number of women significantly under-reported the amount of IFA received.
This combination of over- and under-reporting by different women impacts our ability to accurately estimate coverage of at least 90+ iron-containing supplements. To illustrate, if true coverage increases from 30% in 2025 to 70% in 2030, a national survey using these questions would estimate 47.5% in 2025 and 57.5% in 2030. For this reason, DataDENT recommends option 1 any iron-containing supplement as a process indicator for the anemia target but does not recommend option 2 at least 90 iron-containing supplements.
Less is known about the validity of option 3. DataDENT’s ongoing collaboration with GAIN on coverage measurement for large scale food fortification is focused on household-level coverage of fortifiable staples rather than fortification levels in the overall maize or wheat flour supply. The GFDx includes multiple proxy indicators for fortification levels. The validity of these indicators likely varies by country context given the flexibility in how estimates are derived.
Process indicators for the EBF target
The WHO proposed two process indicators for the EBF target: option 1 – percent of caregivers counselled on infant and young child feeding (IYCF) and option 2 – percent of newborns being put to the breast in the first hour after birth. The recommended data source for option 1 is the UNICEF NutriDash survey which compiles program data and option 2 is the UNICEF IYCF database which includes estimates from Multiple Indicator Cluster Surveys (MICS) and DHS surveys.
WHO proposes using NutriDash data compiled by UNICEF country offices for option 1, but DHS is another potential data source. In our first project cycle, DataDENT spearheaded development of IYCF counseling coverage indicators which were taken up in the DHS-8 core household survey questionnaire. We also contributed to a validation study of community-based IYCF counseling coverage led by IFPRI India. The study team observed and documented counseling messages shared by community health workers during home visits to more than 400 mothers with children less than 1 year old. Two weeks after the observation they returned to ask standard questions about receipt of the IYCF counseling intervention. Women responded with ‘moderate’ accuracy about whether they received any IYCF counseling and about specific breastfeeding, EBF, and dietary diversity messages. Study authors cautioned that recall over periods longer than 2 weeks may not be valid. However, they did recommend using these survey questions to monitor population-level trends in IYCF counseling coverage.
For option 2, DHS and MICS include a question about how long after their most delivery (i.e. last 2-3 years) a woman started breastfeeding. Results from three separate indicator validation studies in Kenya, Mozambique and Mexico, show that the questions over-estimate the true prevalence of early breastfeeding initiation by 25% to 394%. Study authors do not recommend these questions for inclusion in DHS or MICS. Findings from cognitive testing in Ethiopia confirmed that the current questions do not elicit accurate response about initiation of breastfeeding. The Ethiopia study authors recommended significant edits to the questions and using post-delivery exit-interviews for data collection.
Given the consistent findings of low validity for Option 2 early initiation of breastfeeding, DataDENT recommends adopting process indicator Option 1 IYCF counseling coverage. We suggest that WHO includes both program data and national survey data sources.
Invitation for feedback
What do you think about including the validity of estimates as a criterion for selection of WHA nutrition target process indicators? We welcome your questions and comments about DataDENT’s recommendations and encourage you to provide feedback directly into the WHO review process: https://bit.ly/3wWKmyS.
